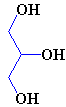
 | Glycerol |
Glycerol is propane which has three -OH groups added, so making it a triol (its correct name is actually 1,2,3-propanetriol). These produce a great deal of intermolecular hydrogen bonding, and as a result glycerol is a viscous, syrupy liquid. Because of this, it is often used in cosmetics, pharmaceuticals and food. In cosmetics it is used to soften and smooth the preparation, in for example creams and pastes. It is also found in toothpaste as an antidrying medium since it hydrogen-bonds strongly to water molecules, preventing them from evaporating. This water-trapping feature is also utilised for the preservation of medical specimens, since the glycerol penetrates the cells and so preserves them without drying them out. Because its structure resembles those of sugars, glycerol has a slightly sweet taste, and this, combined with its viscosity, makes it a desirable component of some wines, adding to their flavour and body. Glycerol is also found in inks, tobaccos, cream-filled sweets, and plastic clays. It is also a component of some plastics, and is used in surface coatings and in the manufacture of synthetic fibres.
 | Glycerol |
Glycerol itself is non-toxic, and is a component of many creamy foodstuffs. However, when heated to high temperatures, glycerol forms acrolein, a colourless liquid which boils at 52°C and whose vapours are irritating to the nose and eyes. Acrolein is responsible for the unpleasant odour of burned oil or fat, and is often detected in the smell of barbecue smoke. Acrolein is also irritating to the digestive tract and may be responsible for the stomach upset caused occasionally by deep-fried foods.
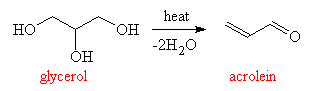
Glycerol is extremely important biologically, since the three -OH groups can react with the acid group of long chained fatty acids in the body. This results in the glycerides (mono-, di-, or tri-glycerides, depending upon whether 1, 2 or 3 of the -OH groups are used), which are better known as animal and vegetable oils and fats. An example is tristearin, which found in beef and cocoa butter (chocolate).
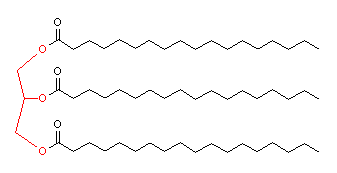 | Tristearin - the glycerol component is highlighted in red. |
 | The insulating blubber of this sperm whale is composed mainly of fats made from triglycerides. |
Glycerol is also important as the main ingredient of nitroglycerin, the powerful explosive which forms the basis of dynamite. Nitroglycerin is made by (very carefully!) reacting glycerol with nitric and sulphuric acid.
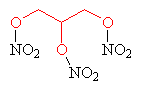 | Nitroglycerin - the glycerol component is highlighted in red. |