 |
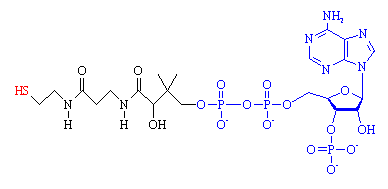
| Coenzyme A. The 'business end', the -SH group where the acetyl group attaches is shown in red. The fragment from ADP is shown in blue. |
In 1945 two biochemists, Lynen and Lipman were investigating the mechanism by which glucose foods are metabolised in the body, and turned either into fats for storage, or energy for immediate use. They found that one of the central molecules involved in this process is a coenzyme (a molecule that helps an enzyme), which they named coenzyme A (or CoA for short). The A stood for acetyl, since one of its main jobs is to transfer two-carbon units in the form of acetyl between various biological molecules.
 |
| Coenzyme A. The 'business end', the -SH group where the acetyl group attaches is shown in red. The fragment from ADP is shown in blue. |
CoA is composed of two main parts, a long protein-like chain (shown in black in the figure), joined to adenosine diphosphate, ADP, (shown in blue) which is one of the molecules used for energy storage. The important part of the molecule is at the end of the protein chain, which terminates in a sulph-hydryl (-SH) group (red). This group is highly reactive, and links to carboxylic acid molecules via a thioester bond. The most important acid is acetic acid, and when it is joined to CoA, the resulting compound is known as acetyl-CoA.
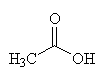 | Acetic acid - the C2 carbon unit that joins to CoA |
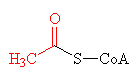 | The thioester bond, joining together acetyl (shown in red) and CoA to make acetyl-CoA. |
The thioester link, however, is very high energy bond, and therefore unstable. This means that the acetyl group can be easily transferred to any other waiting molecule, and so acetyl-CoA is used as a universal intermediate which provides the C2 fragment for numerous syntheses.
CoA is an extremely important biological molecule which is right at the hub of carbohydrate metabolism. This process is called the Citric Acid Cycle because citric acid (or more precisely, the citrate ion) is one of the key chemicals in the series of rections).
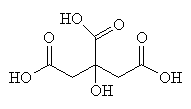 | Citric acid - an important intermediate in the Citric Acid Cycle |
In this mechanism, the C2 fragments resulting from the breakdown of various carbohydrates, fats and proteins, are reacted with CoA, to form acetyl-CoA. The acetyl residue is then transported to other molecules, where it is released and, in the presence of O2, oxidised to carbon dioxide. This generates the energy that cells require for biochemical processes, and the waste CO2 is transported in the bloodstream to the lungs and exhaled. The cycle also produces hydrogen atoms, which then continue in another series of biochemical reactions to produce adenosine triphosphate (ATP) and water. ATP is the molecule which stores energy, in the form of phosphate bonds, for later use, and in the citric acid cycle one molecule of acetyl-CoA generates 12 ATPs. When the acetyl is released, CoA is regenerated, which returns to the citric acid cycle to catalyse another reaction.
If the C2 unit is not to be oxidised to CO2 and the energy used immediately, another possibility is that it can be used to build important biological molecules. Acetyl-CoA is the starting point for the synthesis of isoprenoid molecules, and fatty acids. The latter path is especially important in the transformation of carbohydrates into fat. Acetyl-CoA is also used in the synthesis of esters and amides (e.g. acetylcholine - an important neurotransmitter), and some steroids, (e.g, cholesterol).